Brain disorders constitute the largest socioeconomic burden in Western societies and accordingly, identification of brain disease mechanisms and prediction of treatment outcome is of paramount socio-economic importance. Experimental and translational medicine models are required to identify basic disease mechanisms and to facilitate novel intervention strategies and to prevent or cure brain disorders. This is essential to bridge the gap between animal and human studies and to help mitigate and manage the risk of novel treatments, be it pharmacological or non-pharmacological strategies
The aim of NeuroPharm is to develop and validate new human experimental medicine models in brain disorders in order to identify ways to safely assess novel treatments and intervention outcomes in humans. This will be done by studying brain neurobiology and the brain’s response to neuropharmacological interventions.
The research in NeuroPharm is divided into four work packages (NP1-4) and the specific scientific objectives of these are to:
- predict the outcome of a pharmacological intervention (NP1)
- assess novel treatment interventions for brain disorders (NP2)
- develop and validate neuroimaging methods for experimental medicine approach (NP3)
- build statistical and predictive models of outcomes (NP4)
By means of positron emission tomography (PET) and magnetic resonance (MR) brain scanning we will image brain receptors, receptor occupancy, and the brains regional interactions, i.e., functional connectivity. The ability to simultaneously measure drug occupancy and brain reactivity directly in humans provides a completely novel approach to assess interventional effects. We will employ these brain imaging tools in patients with, e.g., depression and migraine. We will make use of existing data and biological samples to be analysed in the context of a multivariate data analysis framework. Generation of predictive statistical models will allow for a more informed use of data acquired within the Center and will provide a foundation for better study designs. Through this research, we expect to answer pertinent and basic questions regarding human brain disease mechanisms and predict brain responses to categories of neuromodulatory interventions as well as treatment efficacy.
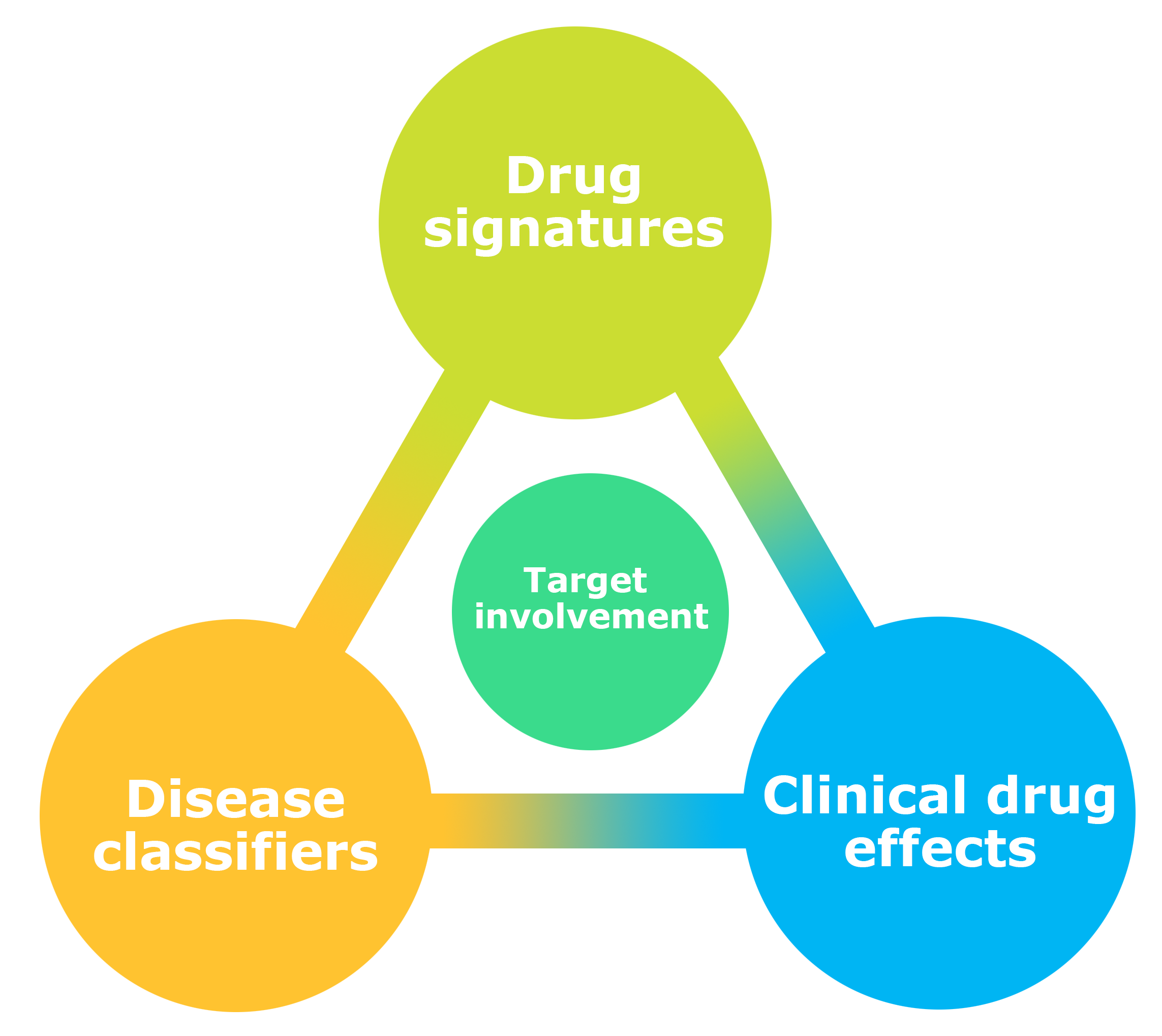
The diagram to the right summarizes the overall framework of NeuroPharm, in the context of drug assessments where the main goal is to identify target involvement. Based on data outcome from the various methods available within the Center, e.g., brain imaging techniques we will gain novel insight into the specific patterns characteristic for individual brain-targeting drugs (Drug signatures). The same endophenotype data can also critically aid to stratify patients and categorize disease subtypes (Disease classifiers) which will enhance prediction of treatment efficacy (Clinical drug effects). Conversely, individuals’ clinical outcome after drug intervention can itself be part of the drug signature and can help inform the disease classification.
The vision of NeuroPharm is to establish itself as an internationally leading hub for experimental medicine in brain disorders.